Home / News / World News / Article /
FDA puts a halt on Covid shots for kids under five
Updated On: 13 February, 2022 09:20 AM IST | Washington | Agencies
The Food and Drug Administration is worried about the toll of Omicron variant on kids
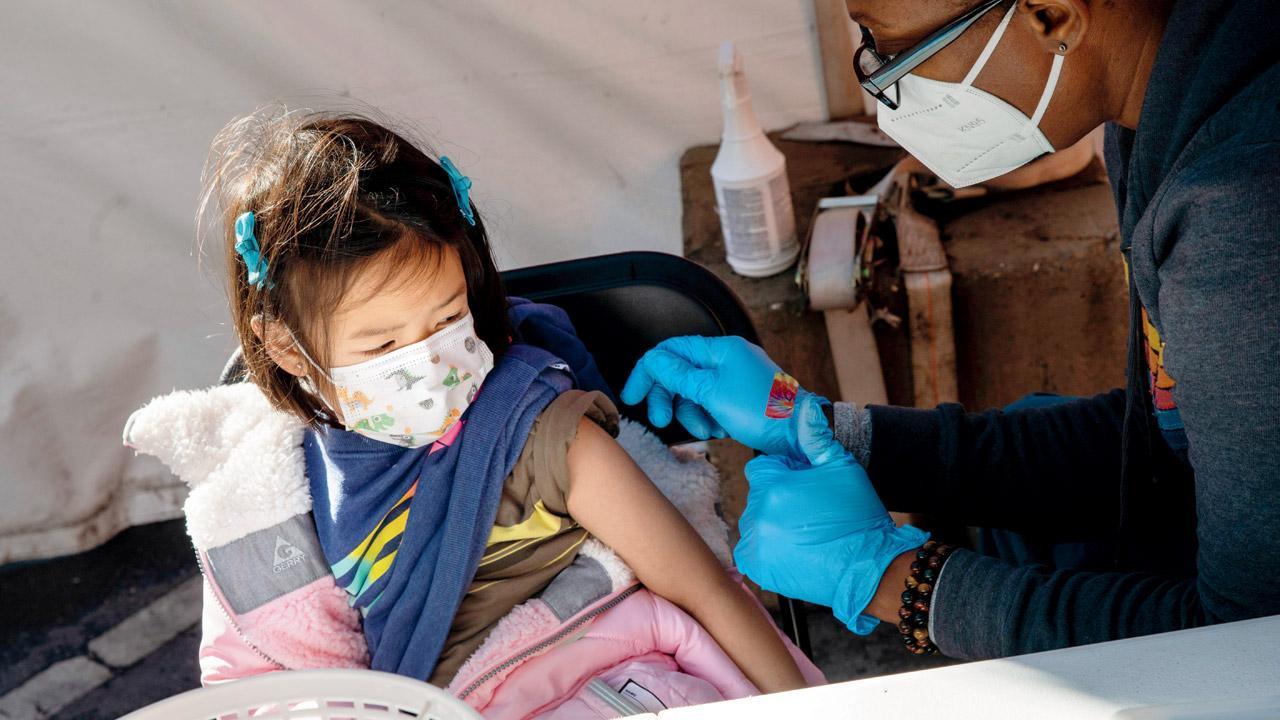
A six-year-old girl gets the Pfizer Covid-19 vaccine in San Francisco. Pic/Getty Images
Covid-19 vaccinations for children under five hit another month-long delay as US regulators abruptly put the brakes on their efforts to speed review of the shots that Pfizer is testing for youngsters. The Food and Drug Administration, worried about the Omicron variant’s toll on kids, had taken the extraordinary step of urging Pfizer to apply for okay of the extra-low dose vaccine before it’s clear if tots will need two shots or three. The agency’s plan could have allowed vaccinations to begin within weeks.
But the FDA reversed course and said it had become clear the agency needed to wait for data on how well a third shot works for the youngest age group. Pfizer said in a statement that it expected the data by early April. FDA’s vaccine chief Dr Peter Marks said he hoped parents would understand that the agency’s decision was part of its careful scientific review of the evidence Pfizer has submitted so far.



